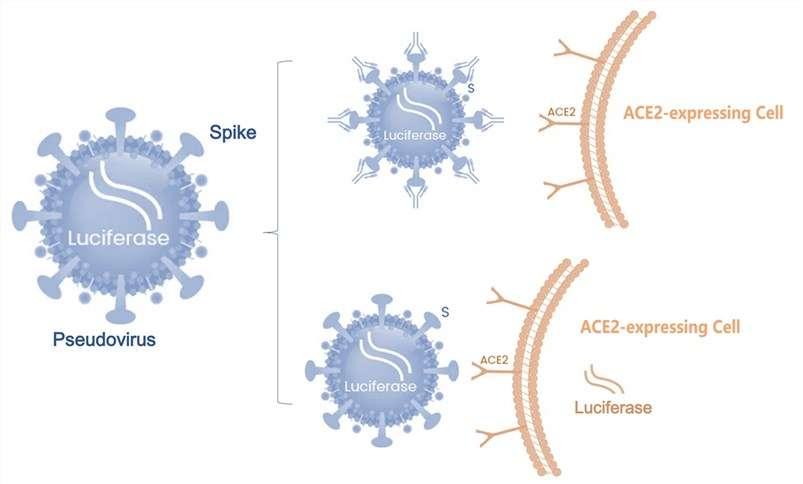
The COVID-19 pandemic, caused by the persistent SARS-CoV-2 virus, has spanned over four years, with evolving variants sparking global outbreaks. To effectively mitigate the ongoing crisis, the acceleration of global vaccination remains a paramount strategy. Speculations among scientists suggest that the novel coronavirus might become endemic, establishing a long-term coexistence with humans. In addition to the regular administration of COVID-19 vaccines, the exploration of broad-spectrum protection represents another avenue for future vaccine development.
Various vaccines for SARS-CoV-2 are currently in the development stage, encompassing inactivated vaccines, live attenuated vaccines, viral vector vaccines, virus-like particle vaccines, subunit vaccines, and nucleic acid vaccines (DNA or RNA vaccines). Despite numerous candidates, only a select few have progressed to human trials. The experiences gained from vaccine research on SARS-CoV-2, both in terms of development methods and safety considerations, have significantly influenced and expedited the development of COVID-19 vaccines.
In the United States, at the onset of the pandemic, two mRNA vaccines and one adenovirus vector vaccine received emergency use authorization (EUA).
Viral vector-based vaccines, sharing antigenic similarities with the pathogen, elicit a robust immune response. However, their preparation is intricate, and vaccine efficacy may be compromised due to pre-existing immunity. Various viral vector platforms, such as adenoviruses (human and non-human primates), modified vaccinia virus Ankara (MVA), Newcastle disease virus (NDV), measles virus, and vesicular stomatitis virus (VSV), have been employed in the development of SARS-CoV-2 vaccines.
Live attenuated vaccines reduce the virus's pathogenicity by altering the viral genome. They contain all the immunogenic components of the original virus, simulating a natural immune system response. Risks associated with live attenuated vaccines include the potential for reversion to high pathogenicity and residual weakened virulence causing harm in immunocompromised individuals. Advances in biotechnology now enable the optimization of influenza and dengue virus pathogenicity through codon deoptimization. Companies like Codagenix and the Serum Institute of India are utilizing this method to reduce the pathogenicity of SARS-CoV-2 in clinical phase I trials in the UK.
While the safety and efficacy of COVID-19 candidate vaccines are generally produced using processes similar to those for SARS-CoV-2, finding the right balance between effectiveness and safety presents a challenge. Animal studies on previous SARS-CoV-2 vaccine development have indicated that vaccination might exacerbate lung inflammation caused by viral infection.
The global persistence of the novel coronavirus pandemic necessitates the urgent acceleration of global vaccination efforts. Alongside the rapid development of more effective vaccines, assessing whether COVID-19 vaccines can induce long-term immunity and broad-spectrum protection becomes a crucial consideration. Continuous observation and data collection on the cross-protective efficacy and safety of vaccines are imperative due to ongoing mutations of the novel coronavirus, especially as vaccination rates increase. Valuable lessons learned from controlling this pandemic should guide preparations for future changes in epidemic situations.